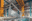What does anodized actually mean
Corrosion protection usually means applying an additional coating. With aluminum, however, a little oxygen is enough. In the electrolysis process, the metal forms a surface of aluminum oxide that is as hard as it is durable. And it can even be colored.
Text: Stephan Becker
Aluminum is a strange material. On the one hand, not particularly valuable when you think of packaging or old GDR coins. On the other hand, it is hard to imagine many high-tech areas without it. Whether in automotive or aircraft construction, whether for lights or high-end laptops, nothing works without aluminum. This is not least due to its lower density compared to steel. Although it requires more volume to achieve a similar level of strength, it is also significantly lighter.
One disadvantage, however, is its chemical reactivity in addition to the greater energy required to produce it. A natural oxidation layer forms quickly, which in contrast to iron oxide - i.e. rust - already has a certain protective effect. However, as a base metal, aluminum is anything but permanently resistant to corrosion. However, its reactivity can be turned to advantage in the anodizing process.
Anodizing stands for the electrolytic oxidation of aluminium. The principle was invented at the beginning of the last century and popularized under this name by the Vereinigte Aluminium-Werke in Germany from the mid-1920s. Using direct current, a significantly thicker oxide layer is produced in an acidic electrolysis bath. And this is characterized by a dramatically improved hardness and protective effect. This makes anodized surfaces usable and hard-wearing for consumer goods.
The fine metallic structure of the surface is visually appealing. Whether shiny or matt depends on the pre-treatment of the workpieces using various stains. And it can finally become colorful after oxidation. Due to the porosity of the crystal structure, simple colorants and a final sealing in a hot water bath are sufficient. Like this last step, all other substances used are based on water-soluble, largely harmless chemicals. Existing anodized layers can be removed with similar ease during recycling. The result of the process is a surface protection that retains the metallic character of the material, even with colored finishes. And because - unlike with a paint or powder coating - nothing is added, only the existing surface is transformed, nothing can flake off, even in the event of mechanical damage.
Pictures: Markus Bredt; Erdoganlar Aluminum
What does that actually mean...?
Raw, unadorned, radical - galvanized surfaces have a special aesthetic and often develop a fascinating beauty in combination with the material, shape and surroundings. At MAGAZIN, we value the material and use it in various products.
Durable, versatile, low-emission, efficient. Powder coating may not sound so glamorous, but if you want a colorful finish, there is hardly a better process for surface protection. Thanks to electrostatic effects, perfect surfaces can be produced. And when the going gets tough, they show character.